Laser Spectroscopy for Isotopic Analysis
Contact Us
- Group Leader
- Craig Taylor
- Deputy Group Leader
- Lance Green
- Acting Deputy Group Leader
- Angela Olson
- Deputy Group Leader
- Stephen Willson
- Group Office
- (505) 667-4087
Atomic Beam Absorption Spectroscopy for Isotopic Analysis in the Field
Principal Investigator: Alonso Castro, Ph.D.
LA-UR-21-20663
The isotopic analysis of nuclear materials in the field has important applications in nuclear forensics for non-proliferation. In the area of safeguards, the rapid detection of isotopic signatures and ratios of uranium and plutonium reveals whether or not nuclear materials are as declared for a given facility. In the case of post-detonation debris, the analysis provides information about the origin, processing history, and composition of the fuel prior to detonation, and also the overall fission efficiency of the device. Mass spectrometry remains the gold standard for high-precision, high-sensitivity isotopic analysis of nuclear materials, but its complexity, sample preparation requirements, and utility requirements (e.g. voltage/power, high vacuum, compressed gases, strong acids) make it unsuitable for deployment in the field.
We have developed new methods and instrumentation for the isotopic analysis of actinides in the fixed-lab and in the field. The method is based on performing laser absorption spectroscopy through a collimated atomic beam of the actinide generated by a high-temperature micro-crucible in rough vacuum. Tantalum micro-crucibles are resistively-heated to temperatures up to 2500 °C. A laser beam from a narrow-linewidth diode laser is tuned across a series of specific isotopic resonances, and the resulting spectrum shows their relative abundance.
Experimental setup for the isotopic analysis of actinides by laser spectroscopy.
In the case of uranium, for example, we have conducted a series of experiments for the determination of the isotopic composition of a variety of samples of uranium metal, oxides, nitrates, halides, including UF6 gas. These samples vary in 235U level of enrichment from natural uranium (0.7%) up to 93%. Excellent signal-to-noise-ratios (up to a few 1000's) are obtained in only a few seconds. By taking the ratio of the areas under each isotope absorption band, the 235U level of enrichment is obtained. The level of enrichment was obtained with an accuracy of 0.2 - 0.4% and a precision of 0.5 - 0.7%. When analyzing oxides and other compounds, the atomic beam is chemically reduced on the fly by addition of lutetium metal to the crucible prior to heating.
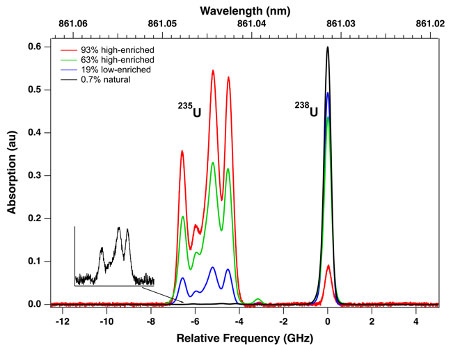
Isotopically-resolved spectra of uranium at various levels of enrichment
We are also in the process of building various fieldable prototypes based on our technique. These instruments are portable, easy to operate, and consume less than 1000 watts of electrical power. The figure below shows the current version of the prototype.

Fieldable prototype instrument for isotopic analysis.
- Lebedev, J. H. Bartlett, A. Malyzhenkov, and A. Castro, "Micro-channel array crucible for isotope-resolved laser spectroscopy of high-temperature atomic beams," Rev. Sci. Instrum. 88, 126101 (2017).
- Lebedev, J. H. Bartlett, and A. Castro, “Isotope-resolved atomic beam laser spectroscopy of natural uranium," J. Anal. At. Spectrom. 33, 1862 (2018).
- H. Bartlett and A. Castro, "Isotopic spectroscopy of uranium atomic beams produced by thermal reduction of uranium compounds," Spectrochim. Acta B 155, 61 (2019).
- Wei, I. Savukov, and A. Castro, "Hyperfine structure studies of the neutral uranium transition at 861.031 nm by saturation absorption spectroscopy," submitted to J. Anal. At. Spectrom.