Chemistry Overview
Pursuing a variety of surface modifications
Contact
- Aaron Anderson
Sensor platforms with thin films
We currently pursue a variety of surface modifications, the most important of which is the attachment of thin films to our sensor platforms and the subsequent attachment of ligands to these surfaces.
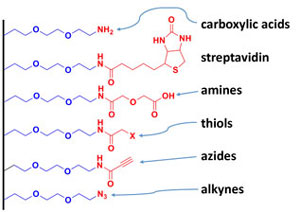
We also make efforts to develop novel silanes and thiols for coating planar surfaces, quantum dots, SERS particles, and silica-based reporters for biofouling resistance, photostability, aggregation prevention, and/or ligand attachment.

Finally, we are working to apply these thin films to other sensing platforms. To reach these goals, we rely on our teammates, surface chemistry/self-assembly techniques, synthetic organic and inorganic chemistry tools, and a variety of analytical methods.
In any detection method, an analyte must interact with a surface. This interaction leads to the translation of a molecular recognition event that we cannot see (binding) into something observable (transduction), such as light emission or an electrical impulse. Therefore, the surface of the transducer plays a critical role in successful detection.
Making the surface of a device useful almost always requires some modification. Most modifications rely on self-assembly and deposition of a precursor to form a thin film; the resulting surface coating is frequently referred to as a self-assembled monolayer (SAM). A useful SAM for biological detection must combine resistance to protein absorption (prevention of non-specific binding) with the ability to intentionally attach ligands or receptors to a surface (enhance specific binding).
The LANL biosensor team has relied on two types of self-assembled systems to modify surfaces: phospholipid bilayers, which mimic cell membranes that are found in nature and are held to a surface by polar interactions; and organo-silanes, which form a covalent bond to a surface through a silane. Both are useful on a variety of oxide surfaces, are highly resistant to non-specific binding, and are modifiable to allow specific binding to occur. Phospholipid bilayers have the advantage of allowing ligand mobility where it is necessary, and are therefore still useful in some applications, but they do not endure long-term (>1 month) storage, treatment with storage in air, or exposure to detergents or high-salt solutions. Furthermore, the modifications available in phospholipids are limited.
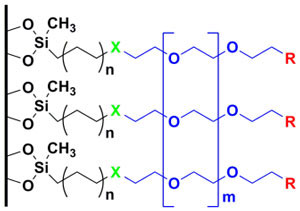
Most biosensor applications require long-term storage of a sensor element, long-term deployment of a device for continuous sampling and detection, and/or processing of complex samples. To address these needs, a variety of methods have been developed, which rely on the reaction of a silane with reactive species (hydroxyl molecules) on the surface of the transducer. Many teams use high molecular weight polyethylene glycol (PEG)-containing silanes to prevent non-specific binding, or small reactive silanes for ligand attachment. These coatings either provide sites for ligand attachment or resistance to biofouling, but rarely achieve both goals.
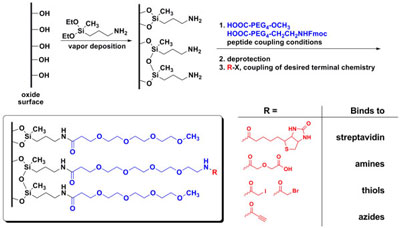
Attachment of thin films to our sensor platforms: Our team has developed a novel thin film that allows specific attachment of ligands to a surface while preventing non-specific binding. To this end, aminopropyldiethoxymethyl silane was attached to a silica surface through a vapor deposition process. This vapor deposition process is at the heart of our SAM chemistry, as it is rapid, inexpensive, and simple. The aminosilane is modified with commercially available polyethylene glycol (PEG) reagents to create the binding surfaces. These surfaces present a small percentage of a functional group, which is available for binding, and a large percentage of molecules that are highly inert to chemical reaction or biological interaction. The films are characterized by fluorescence microscopy, atomic force microscopy, water contact angle and ellipsometry.
Stability of the thin films was assessed by long-term storage. Contact angle did not change significantly after storing the films for a year in ambient air. Functionally, the thin films were validated using sandwich immunoassays against a variety of targets. Most of the data surrounds detection of Bacillus anthracis protective antigen (PA), but these films have been used in the detection of tuberculosis antigens (LAM and Antigen-85), carcinoembryonic antigen, and human influenza, as well as for multi-analyte detection of PA, B. anthracis lethal factor, and streptavidin in the same assay using different colored quantum dots.